
Raising the Bar for Protocol Development
Protocol Builder® is a cloud-based protocol writing technology that makes it faster and easier to develop investigator-initiated clinical research protocols that meet IRB and regulatory standards. It’s a robust yet simple tool that can be accessed on all devices.
.jpg)

Trusted Protocols
Powered by Biomedical Research Alliance of New York (BRANY), a leader in providing hospitals and medical schools IRB and clinical research services. Protocol Builder makes the process of writing, collaborating, and reviewing protocols more efficient.

Efficient Results
Protocol Builder saves significant time with automation, advanced editing tools, and accuracy checking. The amount of revisions are reduced significantly.
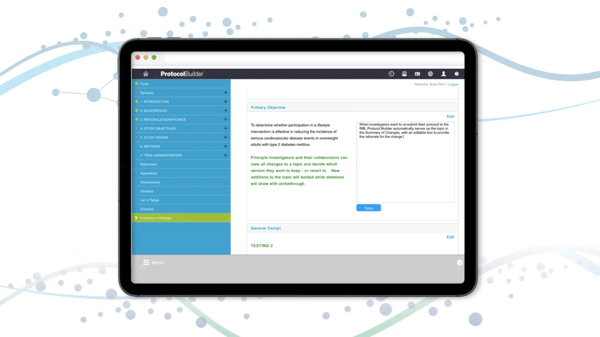
Compliant Standards
Features including expert tips and resources improve adherence to IRB and regulatory standards templates and relevant instructions promote standardization for easier development and review. Tools help determine protocol type and whether an IND/IDE is needed.

Collaborative Approach
Users can invite colleagues and supervisors to comment on or revise protocol topics. Revisions can be tracked, compared, and accepted/rejected. Protocol writers can message reviewers directly about specific changes.
Explore Protocol Builder
Improve protocol completion rates and compliance quality while saving time. Proven to improve adherence to federal and IRB regulations.

Is Protocol Builder Right for You?
-
Research Investigators
Research Investigators

Protocol Builder Develops Protocols Faster
Whether you are an experienced or new research investigator, doctor, medical resident, or medical student, Protocol Builder can help you develop better protocols in less time with less effort, even if you are new to protocol writing. It’s easy to get started and simple to use. It also enables collaboration and helps avoid multiple rounds of revisions, so you can get your research up and running faster.
-
Research Administrators
Research Administrators

Enforce Protocol Standardization with Templates
Protocol Builder can help you enforce protocol standardization with pre-set templates and a guided experience that includes simple instructions and expert tips. It also allows institutions to add specific guidelines and use their own templates in the application. Plus, it offers features to easily compare, track, and summarize protocol revisions. It can be implemented in less than 30 days, and can also be integrated with your IRB, CTMS, or single sign-on platforms.
-
Research Librarians
Research Librarians

Streamline Protocol Writing
This technology can be a valuable addition to your e-resource collection. It streamlines the protocol writing process and promotes learning to support your research, research education, and health science programs. It also helps advance research publication with automated citation/reference functionality and the ability to reference management libraries such as Endnote, RefWorks, Mendeley, and PubMed.
-
Residency Programs
Residency Programs

Develop Consistent Protocols and Learn As You Go
Protocol Builder not only helps residents develop consistent protocols more easily, it also helps them learn as they go. It offers a guided experience developed by protocol writing experts and can bolster your program’s efforts to meet or exceed ACGME research requirements. In addition, it enables communication and collaboration to help foster mentorship.
Research Investigators

Protocol Builder Develops Protocols Faster
Whether you are an experienced or new research investigator, doctor, medical resident, or medical student, Protocol Builder can help you develop better protocols in less time with less effort, even if you are new to protocol writing. It’s easy to get started and simple to use. It also enables collaboration and helps avoid multiple rounds of revisions, so you can get your research up and running faster.
Research Administrators

Enforce Protocol Standardization with Templates
Protocol Builder can help you enforce protocol standardization with pre-set templates and a guided experience that includes simple instructions and expert tips. It also allows institutions to add specific guidelines and use their own templates in the application. Plus, it offers features to easily compare, track, and summarize protocol revisions. It can be implemented in less than 30 days, and can also be integrated with your IRB, CTMS, or single sign-on platforms.
Research Librarians

Streamline Protocol Writing
This technology can be a valuable addition to your e-resource collection. It streamlines the protocol writing process and promotes learning to support your research, research education, and health science programs. It also helps advance research publication with automated citation/reference functionality and the ability to reference management libraries such as Endnote, RefWorks, Mendeley, and PubMed.
Residency Programs

Develop Consistent Protocols and Learn As You Go
Protocol Builder not only helps residents develop consistent protocols more easily, it also helps them learn as they go. It offers a guided experience developed by protocol writing experts and can bolster your program’s efforts to meet or exceed ACGME research requirements. In addition, it enables communication and collaboration to help foster mentorship.
Why Protocol Builder
Protocol Builder offers easy to use features developed by protocol writing and usability experts
-
Automatic Set Up
Simple & Quick Set-Up
Enter basic information, and appropriate protocol sections are set up automatically based on protocol type. Protocol Builder can help you determine the type of study, as well as whether or not you need an IND or IDE application.
-
Protocol Duplication
Stay Efficient
Instead of creating a new protocol, you can duplicate an existing protocol to repurpose the contents and reference
-
Advanced Editing Tools
Better Protocol Building
Insert references, tables, appendices, abbreviations and glossary terms easily as you write. Those sections are then set up automatically at the end of the document.
-
Expert Guidance
Guidance Along the Way
Receive relevant instructions and expert tips as you go through the protocol. You’ll also find sample protocols in the Resource Center that you can use for reference or as a template.
-
Share, Review, Compare Revisions
Collaborate with Peers
Invite others to review and add comments directly into the appropriate sections. Compare revisions made to a particular section. Accept or disregard them.
-
Summary of Changes
Stay Informed of Protocol Updates
Review all recent changes to a protocol and enter a rationale for each one. This information will be used to create a Summary of Changes page, which can be included if a protocol needs to be resubmitted.
-
Reference Management
The Best of Reference Management Integration
Pull in reference management libraries, such as Endnote, RefWorks, Mendeley, and PubMed.
Simple & Quick Set-Up
Enter basic information, and appropriate protocol sections are set up automatically based on protocol type. Protocol Builder can help you determine the type of study, as well as whether or not you need an IND or IDE application.
Stay Efficient
Instead of creating a new protocol, you can duplicate an existing protocol to repurpose the contents and reference
Better Protocol Building
Insert references, tables, appendices, abbreviations and glossary terms easily as you write. Those sections are then set up automatically at the end of the document.
Guidance Along the Way
Receive relevant instructions and expert tips as you go through the protocol. You’ll also find sample protocols in the Resource Center that you can use for reference or as a template.
Collaborate with Peers
Invite others to review and add comments directly into the appropriate sections. Compare revisions made to a particular section. Accept or disregard them.
Stay Informed of Protocol Updates
Review all recent changes to a protocol and enter a rationale for each one. This information will be used to create a Summary of Changes page, which can be included if a protocol needs to be resubmitted.
The Best of Reference Management Integration
Pull in reference management libraries, such as Endnote, RefWorks, Mendeley, and PubMed.
Protocol Builder Materials
Helpful information on Protocol Builder to help you maximize these and all TDS Health resources.
Frequently Asked Questions
If you don’t get an answer to what you’re looking for below, please reach out to TDS Health. We’ll be happy to answer any questions and schedule a free demo.
Q: Can I See How Protocol Builder Works?
Q: How Much Does It Cost?
We offer institutional subscriptions based on approximate users. Contact us to get a formal quote.
Q: I Am a Subscriber, How Do I Log In and Get Trained?

Our investigators really appreciate the step by step guidance, the collaboration tools, and the protocol completeness dashboard features. Our goal is to use Protocol Builder as our protocol writing hub to enhance quality, reduce review time, and improve adherence to federal and IRB regulations.
Clinical Research Navigator Clinical Translational Science Institute at Children’s National (CTSI-CN)

